Multiwfn forum
Multiwfn official website: http://www.shanxitv.org/multiwfn. Multiwfn forum in Chinese: http://bbs.keinsci.com/wfn
You are not logged in.
- Topics: Active | Unanswered
#1 2025-02-04 01:05:59
- Alex679
- Member
- Registered: 2025-02-03
- Posts: 1
ETS-NOCV for a complex bearing chelating ligand
Hello!
I'd like to know how to obtain the orbitals and energies for a complex bearing a chelating ligand. In the manual there are only examples of complexes with monodentate ligands.
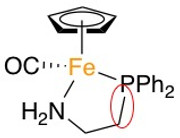
I've attached a picture with the complex that I want to study.
For this complex the problem arise from the study of the Fe-PPh2 bond because this requires the creation of the fragment without PPh2 group and for this purpose, the bond cleavage between PPh2 group and CH2 (link of the hydrocarbon chain of chelating ligand) is also required.
I am just interested in the Fe-PPh2 bond because I want to know how strong this bond is against the Fe-CO bond, but if I create the fragment without PPh2 it also implies the cleavage of the CH2-PPh2 bond and in the final result of the ETS-NOCV calculation I will see the orbitals and energies of both bonds. How could I perform this type of calculation?
Last edited by Alex679 (2025-02-04 03:00:03)
Offline
#2 2025-02-04 07:00:00
Re: ETS-NOCV for a complex bearing chelating ligand
It is not possible to fully avoid the coupling between the two bond-breaking sites. I suggest taking PPh2-CH2 as a fragment, then the two bond-breaking sites will be relatively farther apart. Then when performing ETS-NOCV analysis via Multiwfn, you should find many NOCV pairs are highly localized on the Fe-P bonding region, which are what you should focus on.
Offline